5 latest updates for regulation and safety in beauty: 2019

5. Unilever goes further with enhanced ingredients transparency
At the start of this year, the multinational consumer goods player announced that it successfully delivered on its ‘industry leading’ commitment to voluntarily disclose fragrance ingredients across its home care and beauty & personal care products in Europe and the US by the end of 2018. It also asserted it aims to expand the programme. Discover our full report here.
“The company’s decision to disclose fragrance ingredient information down to 0.01% of the product formulation was an industry first, paving the way and encouraging many other companies to do the same,” suggests Unilever.
4. Cosmetics carcinogens after Brexit? CTPA smacks down suggestion
The UK’s cosmetics trade association has asserted that “cosmetic products will continue to be safe after Brexit”, following media coverage at the start of this year that suggested standards on safety for beauty may slip in the UK once it leaves the EU.
“For any cosmetic product being placed on the UK market, irrespective of its origin, consumers can be reassured that both now and after we leave the EU all ingredients must be safe to use, as must the final cosmetic product.,” the trade association says. Read more here.
3. ‘The future of cruelty-free cosmetics in Europe’ at risk, claims animal rights group
Cruelty Free International, an animal rights group, has suggested the full enforcement of animal testing bans for cosmetics in Europe are under threat.
The group says that the European Chemicals Agency (ECHA) is ‘undermining’ European cosmetics animal testing bans, and “chipping away at the Cosmetics Regulation by asking for animal tests on substances used primarily or exclusively in cosmetics”. Read our full report, including ECHA response, here.
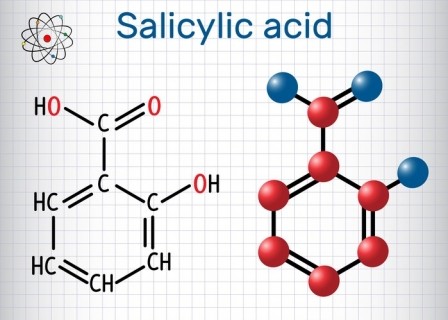
2. EU’s SCCS gives safety nod to Salicylic acid
The European Union’s Scientific Committee on Consumer Safety has given its approval rating for Salicyclic Acid in cosmetic and personal care formulations. Read our full report on the decision here.
In a finnal opinion, scientists at the EU organisation evaluated the Salicyclic Acid’s use as a preservative with a maximum concentration of 0.5%, as well as for non-preservative purposes with a dosing of more than 3.0% for rinse of products and up to 2.0% in other products.
1. New study supports the use of zinc oxide nanoparticles in sunscreens
A study, led by two Australian universities and released at the start of this year, has found evidence that zinc oxide nanoparticles used in sunscreen does not cause cellular toxicity even after repeated applications.
The research found that zinc oxide (ZnO) nanoparticles does not penetrate the skin, refuting claims about the safety of nanoparticulate-based sunscreens. Discover our full report here.















![Dr. G is continuing to expand in South East Asia with a new store in Malaysia. [Dr. G]](https://www.cosmeticsdesign-europe.com/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/business-financial/dr.-g-doubles-down-on-sea-expansion-on-the-back-of-sunscreen-popularity/16498790-1-eng-GB/Dr.-G-doubles-down-on-SEA-expansion-on-the-back-of-sunscreen-popularity.jpg)




